Recently, the research group of Professor Wang Jinlan of Southeast University has achieved new progress in the research of nitrogen-fixing catalyst. The research achievement was published on the top periodical of chemical sciences with the title of “Metal-Free Single Atom Catalyst for N2 Fixation Driven by Visible Light” (Journal of the American Chemical Society).
Nitrogen is one of the most important elements of living beings. Although the nitrogen content in the atmosphere is as high as 78%, the activation of nitrogen is very difficult. At present, the Haber–Bosch method is widely applied to the industry to reduce nitrogen to ammonia; however, this process has to be carried out under high temperature and high pressure, thus the energy consumption is high. Statistics show that the annual energy consumption of ammonia production exceeds 1% of global annual energy consumption. Photo/electrocatalytic nitrogen fixation is a new approach to synthesize ammonia, which can achieve nitrogen reduction under normal temperature and pressure, thus causing widespread concern. The core issue is to seek and design highly efficient, stable and inexpensive catalyst.
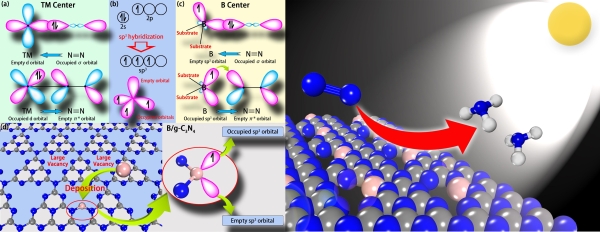
At present, the high-efficiency nitrogen-fixing catalyst is mainly based on the transition metal (TM) compound, and there are few reports on non-metallic catalyst. The coexistence of vacant d-orbital and the occupation ofd electrons in the transition metal can not only accommodate the lone pair of N atoms in the nitrogen molecule, but provide electrons to the anti-bond orbital of to nitrogen molecule, thereby activating the N≡N triple bond and enhancing N?TM button.
By analyzing the extranuclear electronic structure of boron atoms, Professor Wang Jinlan's group found that the sp3 hybrid boron atoms were similar to the transition metals and theseatoms highlighted both empty orbits and occupied orbits; therefore, they were expected to activate and reduce nitrogen. Upon analysis of their structure, performance and other aspects, the group finally chose g-C3N4 as the substrate to support the sp3 doped boron atoms and designed the first metal-free monoatomic catalyst, B/g-C3N4. Theoretical calculations showed that B/g-C3N4 can effectively reduce nitrogen to ammonia by an enzymatic mechanism at a very low initial potential (0.20 V). In addition, the modification of boron can significantly enhance the absorption of visible light of g-C3N4, therefore, it is expected to achieve a solar-driven nitrogen fixation reaction. In addition, such catalyst also features great synthetic prospects and extremely high stability.
The first author of the work is Dr. Ling Chongyi from the School of Physics of Southeast University with Professor Wang Jinlan and Professor Du Aijun from Queensland University of Science and Technology as the corresponding authors.
The above work was funded by the National Outstanding Youth Fund, the National Key R&D Program, the “333 High-Level Talent Cultivation Project” of Jiangsu Province and the National Fund for Overseas Study (School of Physics).
Paper linkage: http://pubs.acs.org/doi/abs/10.1021/jacs.8b07472
















