[Southeast University News Network, Feb. 26] (Correspondent: Xiong Rengen) Recently, the International Institute of Molecular Ferroelectric Science and Application Affiliated to the School of Chemistry and Chemical Engineering of Southeast University and Jiangsu Key Laboratory of “Molecular Ferroelectric Science and Application” have achieved progress in the field of three-dimensional lead halide perovskite ferroelectric, which was published with the title of “A Three-Dimensional Lead Halide Perovskite-Related Ferroelectric” in the “Journal of the American Chemical Society”, the top journal in the field of chemistry.
The organic-inorganic hybrid perovskite materials often have ferroelectricity because of their unique structure. As the inherent spontaneous polarization of ferroelectric materials can be reversed under the action of external electric fields, this has injected new vitality to the new generation of perovskite solar cells. The ferroelectricity photovoltaic effect relies on the polarization to induce the generation of a built-in electric field, thereby promoting photocarrier separation. This characteristic is often accompanied by many optoelectronic phenomena in ferroelectric semiconductors. The three-dimensional organic-inorganic hybrid lead halide perovskite represented by (CH3NH3) PbI3 has been widely concerned because of its functional diversity and broad prospects in the field of optoelectronic application. However, the three-dimensional lead halide perovskite structure is extremely rare, and its ferroelectricity has been under intense debate. Here, the study reported a lead halide ferroelectric with a three-dimensional perovskite structure for the first time, [2-trimethylammonioethylammonium] Pb2Cl6 ([TMAEA] Pb2Cl6) (Fig. 1), with a phase-transition temperature of 421K. At the same time, it is also a high-temperature molecular ferroelectric highlighting excellent semiconducting property and a direct band gap of 3.43 eV.
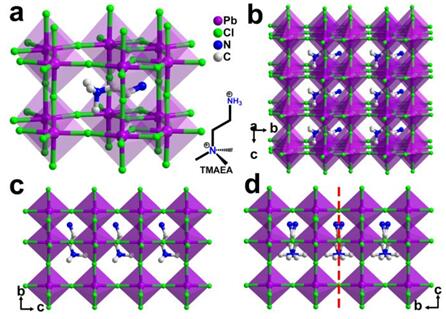
Fig. 1(a, b) Crystal Structure Diagram of [TMAEA] Pb2Cl6 under 293 K; (c) Crystal Stacking Diagram under 293 K; (d) Crystal Stacking Diagram under 293 K
The inorganic ceramic BaTiO3 (BTO) has been widely used because of its excellent structural stability and ferroelectricity. The phase-transition temperature (393 K) of BTO is slightly lower than that of [TMAEA] Pb2Cl6 with and the band gap (~ 3.59 eV) similar to that of [TMAEA] Pb2Cl6. However, the phase-transition mechanism of [TMAEA] Pb2Cl6 (ordered-disordered) is different from that of BTO (shifted), which results in significant gap between the two in terms of saturation polarization value. (Fig. 2) The saturation polarization value of inorganic ceramic BTO is 26 μC/cm2, only 1 μC/cm2 regarding the molecular ferroelectric [TMAEA] Pb2Cl6. Nevertheless, the molecular materials, for their superior structural adjustability, will inevitably bring about more challenges and opportunities after organic combination with chemical design. This research will not only enrich the family of three-dimensional lead halide perovskites, but also guide the study in this regard. All of these efforts have set the keynote for making major breakthroughs in the application of a new generation of organic-inorganic hybrid perovskite based on the ferroelectric heterogeneous photovoltaic effect in the field of photovoltaic devices.
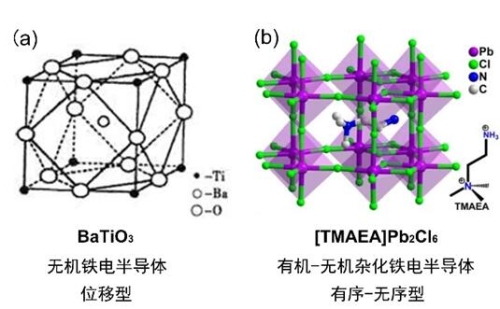
Fig. 2: Schematic Diagram of Crystal Structure: (a) BaTiO3; (b) [TMAEA] Pb2Cl6.
The organic-inorganic hybrid lead halide ferroelectric semiconductor material combines its own ferroelectricity and the semiconductor characteristics with its structural advantages such as low cost, easy processing and mechanical flexibility, etc.. It is expected to provide a better solution for the new generation of flexible optoelectronic material. In view of this, upon unremitting efforts, the International Institute of Molecular Ferroelectric Science and Application Affiliated to the School of Chemistry and Chemical Engineering of Southeast University considered [CH3NH3] PbCl3 of central symmetrical structure as the prototype and utilized Goldschmidt tolerance factor rules to have realized the chemical design of the three-dimensional lead chloride perovskite structure and the perfect combination of ferroelectricity and semiconductor performance in this material. This is the first report of a three-dimensional lead halide perovskite ferroelectric material. Its phase-transition temperature is 407.7 K (much higher than 330K for (CH3NH3) PbI3), which can further guarantee its adaptation to a wider range of applications (Fig. 3). Through the piezoelectric microscope (PFM), we can directly observe the ferroelectric domain structure and its reversal under the action of an external electric field, which can directly demonstrate the ferroelectricity of this material (Fig. 4). All of these efforts have shown huge application potentials of organic-inorganic hybrid lead halide perovskite ferroelectric in the new generation of photovoltaic devices.
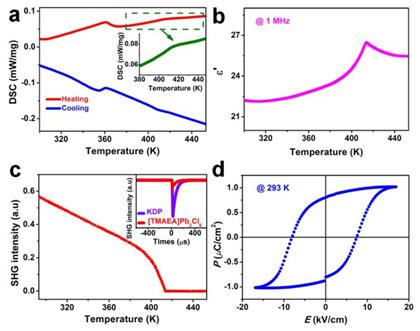
Fig. 3: (a) DSC curve of [TMAEA] Pb2Cl6; (b) the real part curve of dielectric constant changing with the temperature under the frequency of 1 MHz; (c) the intensity of SHG changing with the temperature; the embedded figure: the comparison of SHG intensity between [TMAEA]Pb2Cl6 and KDP at 293 K; (d) P?E hysteresis loop at 293 K.

Fig. 4. Domain structure and polarization reversion diagram of [TMAEA] Pb2Cl6.
Dr. Zhang Hanyue fromthe School of Chemistry and Chemical Engineering of Southeast University is the first author of this paper, and Southeast University is the first corresponding unit. This research results have been sponsored by the launch and cultivation fund of “Top Ten Science and Technology Issues of Southeast University”.
Paper link: https://pubs.acs.org/doi/10.1021/jacs.0c00375
Submitted by: the School of Chemistry and Chemical Engineering
(Editor-in-charge: Ji Hong, reviewed by: Li Xiaonan)
















